Knowledge graphs can support many biomedical applications. These graphs represent biomedical concepts and relationships in the form of nodes and edges. In this review we discuss how these graphs are constructed and applied with a particular focus on the ways machine learning approaches are changing these processes. In many examples in the literature, biomedical knowledge graphs have been constructed from pre-existing databases that are populated by experts via manual curation, but we are now also seeing a more robust use of automatic systems. A number of techniques are used to represent knowledge graphs, but often machine learning methods are used to learn a low-dimensional representation that can support many different applications. This representation is designed to preserve a knowledge graph’s local and/or global structure. Additional machine learning methods can be applied to this representation to make predictions within genomic, pharmaceutical, and clinical domains. We frame our discussion first around knowledge graph construction then around unifying techniques and unifying applications separately. Advances in machine learning for biomedicine are creating new opportunities across many domains, and we note potential avenues for future work with knowledge graphs that appear particularly fruitful.
Knowledge graphs are a practical resource for many real world applications. They have been used in social media mining to classify nodes [1] and create recommendation systems [2]. These graphs have also been used in natural language processing to interpret simple questions and use relational information to provide answers [3,4]. In a biomedical setting these graphs have been used to prioritize genes relevant to disease [5,6,7,8], perform drug repurposing [9] and identify drug-target interactions [10].
Despite their utility, precisely defining a knowledge graph is a difficult task because there are multiple conflicting definitions [11]. For this review, we define a knowledge graph as the following: a resource that integrates single or multiple sources of information into the form of a graph. This graph allows for the capacity to make semantic interpretation, continuously incorporate new information and uncover novel hidden knowledge through computational techniques and algorithms. Based on this definition resources like Hetionet [9] would be considered a knowledge graph as Hetionet integrates multiple sources of information into the form of a graph (example shown in Figure 1). Hetionet was used to derive novel information concerning unique drug treatments [9]. We do not consider databases like DISEASES [12] and DrugBank [13] to be knowledge graphs. Although these resources contain essential information, they do not represent their data in the form of a graph.
In this review we describe various approaches for constructing and applying knowledge graphs in a biomedical setting. We discuss the pros and cons of constructing a knowledge graph via manually curated databases and via text mining systems. We also compare assorted approaches for applying knowledge graphs to solve biomedical problems. Lastly, we conclude on the practicality of knowledge graphs and point out future applications that have yet to be explored.
Knowledge graphs can be constructed in many ways using resources such as pre-exisitng databases or text. Usually, knowledge graphs are constructed using pre-existing databases and these databases are constructed by domain experts using approaches ranging from manual curation to automated techniques, such as text mining. Manual curation is a time consuming process that requires domain experts to read papers and annotate sentences that assert a relationship. Automated approaches rely on machine learning or natural language processing techniques to rapidly detect sentences of interest. We categorize these automated approaches into the following groups: rule-based extraction, unsupervised machine learning, and supervised machine learning and discuss examples of each type of approach while synthesizing their strengths and weaknesses.
Database construction can date back all the way to 1956 where the first database contained a protein sequence of the insulin molecule [21]. This process involves gathering relevant text such as journal articles, abstracts, or web-based text and having curators read the gathered text to detect sentences that implicate a relationship (i.e. relationship extraction). Notable databases constructed by this process can be in found in Table 1. An example database, COSMIC [22] was constructed via a group of domain experts scanning the literature for key cancer related genes. This database contained approximately 35M entries in 2016 [22] and by 2019 had grown to 45M entries [23]. Studies have shown that databases constructed in this fashion contain relatively precise data, but in low quantifies [24,25,26,27,28,29,30]. This happens because the publication rate is too high for curators to keep up [31]. This bottleneck highlights a critical need for future approaches to scale fast enough to compete with the increasing publication rate.
Despite the negatives of manual curation, it is still an essential process for extracting relationships from text. This process can be used to generate gold standard datasets that automated systems use for validation [41,42] and can be used during the training process of these systems (i.e. active learning) [43]. It is important to remember that manual curation alone is precise, but results in low recall rates [30]. Future databases should consider initially relying on automated methods to obtain sentences at an acceptable recall level, then incorporate manual curation as a way to fix or remove irrelevant results.
Rule based-extraction consists of identifying essential keywords and grammatical patterns to detect relationships of interest. Keywords are established via expert knowledge or though the use of pre-existing ontologies, while grammatical patterns are constructed via experts curating parse trees. Parse trees are tree data structures that depict a sentence’s grammatical structure and come into two forms: a constituency parse tree (Figure 2) and a dependency parse tree (Figure 3). Both trees use part of speech tags, labels that dictate the grammatical role of a word such as noun, verb, adjective, etc, for construction, but represent the information in two different forms. Constituency parse trees breaks a sentence down into a subphrases (Figure 2) while dependency path trees analyzes the grammatical structure of a sentence (Figure 3). Many text mining approaches [51,52,53] use such trees to generate features for machine learning algorithms and these approaches are discussed in later sections. In this section we focus on approaches that use rule based extraction as a primary strategy to detect sentences that allude to a relationship.
Pattern matching is a fundamental approach used to detect relationship asserting sentences. These patterns can consist of phrases from constituency trees, a set of keywords or some combination of both [28,59,60,61,62,63]. Xu et al. designed a pattern matcher system to detect sentences in PubMed abstracts that indicate drug-disease treatments [62]. This system matched drug-disease pairs from ClinicalTrials.gov to drug-disease pairs mentioned in abstracts. This matching process aided the authors in identifying sentences that can be used to create simple patterns, such as “Drug in the treatment of Disease” [62], to match other sentences in a wide variety of abstracts. The authors hand curated two datasets for evaluation and achieved a high precision score of 0.904 and a low recall score of 0.131 [62]. This low recall score was based on constructed patterns being too specific to detect infrequent drug pairs. Besides constituency trees, some approaches used dependency trees to construct patterns [51,64]. Depending upon the nature of the algorithm and text, dependency trees could be more appropriate than constituency trees and vise versa. The performance difference between the two trees remains as an open question for future exploration.
Rules based methods provide a basis for many relationship extraction systems. Approaches in this category range from simplifying sentences for easy extraction to identifying sentences based on matched key phrases or grammatical patterns. Both require a significant amount of manual effort and expert knowledge to perform well. A future direction is to develop ways to automatically construct these hand-crafted patterns, which would accelerate the process of creating these rule-based systems.
Unsupervised extractors draw inferences from textual data without the use of annotated labels. These methods involve some form of clustering or statistical calculations. In this section we focus on methods that use unsupervised learning to extract relationships from text.
An unsupervised extractor can exploit the fact that two entities may appear together in text. This event is referred to as co-occurrence and studies that use this phenomenon can be found in Table 2. Two databases DISEASES [12] and STRING [67] were populated using a co-occurrence scoring method on PubMed abstracts, which measured the frequency of co-mention pairs within individual sentences as well as the abstracts themselves. This technique assumes that each individual co-occurring pair is independent from one another. Under this assumption mention pairs that occur more than expected were presumed to implicate the presence of an association or interaction. This approach was able to identify 543,405 disease gene associations [12] and 792,730 high confidence protein protein interactions [67], but is limited to only using PubMed abstracts.
Unsupervised extractors often treat different biomedical relationships as multiple isolated problems. An alternative to this perspective is to capture all different types at once. Clustering is an approach that accomplish this concept of simultaneous extraction. Percha et al. used a biclustering algorithm on generated dependency parse trees to group sentences within PubMed abstract [70]. Each cluster was manually curated to determine which relationship each group represented. This approach captured 4,451,661 dependency paths for 36 different groups [70]. Despite the success, this approach suffered from technical issues such as dependency tree parsing errors. These errors resulted in some sentences not being captured by the clustering algorithm [70] and future clustering approaches should consider simplifying sentences to prevent this type of issue.
Overall unsupervised methods provide a means to rapidly extract relationship asserting sentences without the need of annotated text. Approaches in this category range from calculating co-occurrence scores to clustering sentences and provide a generalizable framework that can be used on large repositories of text. Full text has already been show to meaningfully improve the performance of methods that aim to infer relationships using cooccurrences [68], and we should expect similar benefits for machine learning approaches. Furthermore, we expect that simplifying sentences would improve unsupervised methods and should considered as an initial preprocessing step.
Supervised extractors use labeled sentences to construct generalized patterns that bisect positive examples (sentences that allude to a relationship) from negative ones (sentences that do not allude to a relationship). Most of these approaches have flourished due to pre-labelled publicly available datasets (Table 3). These datasets were constructed by curators for shared open tasks [76,77] or as a means to provide the scientific community with a gold standard [77,78,79]. Approaches that use these available datasets range from using linear classifiers such as support vector machines (SVMs) to non-linear classifiers such as deep learning techniques. The rest of this section discuss approaches that use supervised extractors to detect relationship asserting sentences.
Some supervised extractors involve mapping textual input onto a high dimensional space. SVMs are a type of classifier that can accomplish this task with a mapping function called a kernel [53,80]. These kernels take information such as a sentence’s dependency tree [51,52], part of speech tags [53] or even word counts [80] and map them onto a dense feature space. Within this space, these methods construct a hyperplane that separates sentences in the positive class (illustrates a relationship) from the negative class (does not illustrate a relationship). Kernels can be manually constructed or selected to cater to the relationship of interest [52,53,80,80]. Determining the correct kernel is a nontrivial task that requires expert knowledge to be successful. In addition to single kernel methods, a recent study used an ensemble of SVMs to extract disease-gene associations [81]. This ensemble outperformed notable disease-gene association extractors [64,82] in terms of precision, recall and F1 score. Overall, SVMs have been shown to be beneficial in terms of relationship mining; however, major focus have shifted to utilizing deep learning techniques as these approaches can perform non-linear mappings of high dimensional data.
Deep learning is an increasingly popular class of techniques that can construct their own features within a high dimensional space [83,84]. These methods amount to different forms of neural networks, such as recurrent or convolutional neural networks, to perform classification.
Recurrent neural networks (RNN) are designed for sequential analysis and use a repeatedly updating hidden state to make predictions. An example of a recurrent neural network is a long short term memory (LSTM) network [85]. Cocos et al. [86] used a LSTM to extract drug side effects from de-identified twitter posts, while Yadav et al. [87] used an LSTM to extract protein-protein interactions. Others have also embraced LSTMs to perform relationship extraction [86,88,89,90,91]. Despite the success of these networks, training can be difficult as these networks are highly susceptible to vanishing and exploding gradients [92,93]. One proposed solution to this problem is to clip the gradients while the neural network trains [94]. Besides the gradient problem, these approaches only peak in performance when the datasets reach at least a tens of thousand of data points [95].
Convolutional neural networks (CNNs), which are widely applied for image analysis, use multiple kernel filters to capture small subsets of an overall image [84]. In the context of text mining an image is replaced with words within a sentence mapped to dense vectors (i.e., word embeddings) [96,97]. Peng et al. used a CNN to extract sentences that mentioned protein-protein interactions [98] and Zhou et al. used a CNN to extract chemical-disease relations [99]. Others have used CNNs and variants of CNNs to extract relationships from text [100,101,102]. Just like RNNs, these networks perform well when millions of labeled examples are present [95]; however, obtaining these large datasets is a non-trivial task. Future approaches that use CNNs or RNNs should consider solutions to obtaining these large quantities of data through means such as weak supervision [103], semi-supervised learning [104] or using pre-trained networks via transfer learning [105,106].
Weak or distant supervision takes a different approach that uses noisy or even erroneous labels to train classifiers [103,108,109,110]. Under this paradigm sentences are labeled based on their mention pair being present (positive) or absent (negative) in a database and, once labeled, machine learning classifier can now be trained to extract relationships from text [103]. For example, Thomas et al. [111] used distant supervision to train a SVM to extract sentences mentioning protein-protein interactions (ppi). Their SVM model achieved comparable performance against a baseline model; however, the noise generated via distant supervision was difficult to eradicate [111]. A number of efforts have focused on combining distant supervision with other types of labeling strategies to mitigate the negative impacts of noisy knowledge bases [112,113,114]. Nicholson et al. [102] found that, in some circumstances, these strategies can be reused across different types of biomedical relationships to learn a heterogeneous knowledge graph in cases where those relationships describe similar physical concepts. Combining distant supervision with other types of labeling strategies remains an active area of investigation with numerous associated challenges and opportunities. Overall, semi-supervised learning and weak supervision provide promising results in terms of relationship extraction and future approaches should consider using these paradigms to train machine learning classifiers.
Knowledge graphs can help researchers tackle many biomedical tasks such as finding new treatments for existing drugs [9], aiding efforts to diagnose patients [119] and predicting associations between diseases and biomolecules [120]. In many cases, solutions rely on representing knowledges graphs in a low dimensional space, which is a process called representation learning. This space preserves a knowledge graph’s local and/or global structure and can support efforts to apply machine learning methods to make predictions. In the next sections we review the unifying techniques that construct this low dimensional space and unifying applications that use this space to solve biomedical problems.
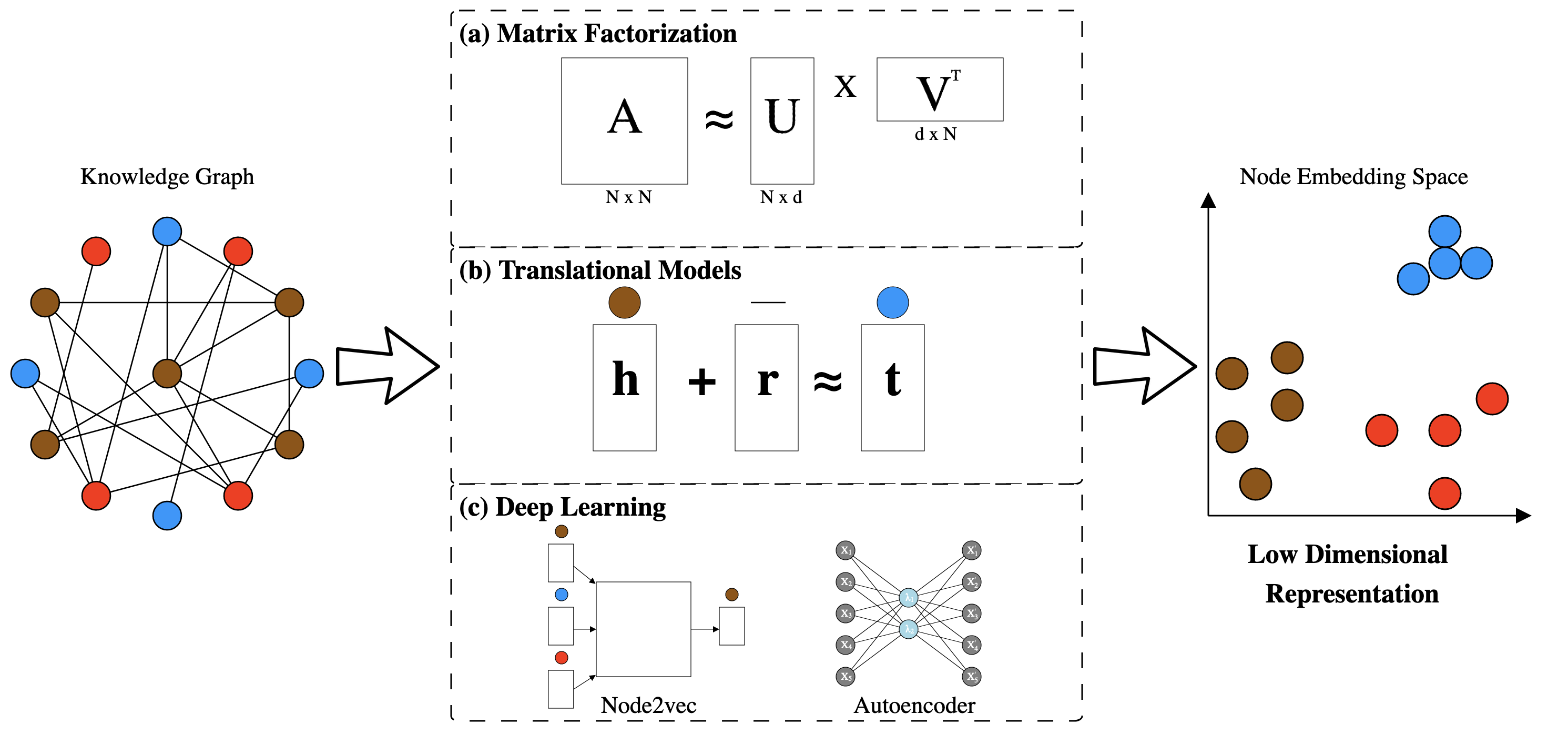
Mapping high dimensional data into a low dimensional space greatly improves modeling performance in fields such as natural language processing [96,97] and image analysis [121]. The success of these approaches provides rationale for representing knowledge graphs into a low dimensional space [122]. Techniques that construct this representation often require information on how nodes are connected with one another [123,124,125,126], while other approaches can work directly with the edges themselves [127]. We group these methods into the following three categories: matrix factorization, translational methods, and deep learning (Figure 4).
Matrix factorization is a technique that uses linear algebra to map high dimensional data onto a low dimensional space. This projection is accomplished by decomposing a matrix into a set of small rectangular matrices (Figure 4 (a)). Notable methods for matrix decomposition include Isomap [128], Laplacian eigenmaps [129] and Principal Component Analysis (PCA) [130]/Singular Vector Decomposition (SVD) [131]. These methods were designed to be used on many different types of data; however, we discuss their use in the context of representing a knowledge graphs in a low dimensional space.
Laplacian eigenmaps is a technique that assumes there is low dimensional structure in a high dimensional space [129]. This algorithm preserves this structure while projecting data into a low dimensional space. Typically, the first step of this algorithm is to construct a figurative knowledge graph where nodes represent datapoints and edges are constructed based on similarity of two datapoints; however, in this context, the knowledge graph has already been defined. The next step in this algorithm is to obtain both an adjacency matrix (\(A\)) and a degree matrix (\(D\)) from the knowledge graph. A degree matrix is a diagonal matrix where each entry represents the number of edges connected to a node. The adjacency and degree matrices are converted into a laplacian matrix (\(L\)), which is a matrix that shares the same properties as the adjacency matrix. The laplacian matrix is generated by subtracting the adjacency matrix from the degree matrix (\(L=D-A\)) and, once constructed, the algorithm uses linear algebra to calculate the laplacian’s eigenvalues and eigenvectors (\(Lx = \lambda Dx\)). The generated eigenvectors represent the knowledge graph’s nodes represented in a low dimensional space [129]. Other efforts have used variants of this algorithm to construct their own low dimensional representations of knowledge graphs [123,124,135]. Typically, eigenmaps work well when knowledge graphs have a sparse number of edges between nodes but struggle when presented with denser networks [134,135,136]. An open area of exploration is to adapt these methods to accommodate knowledge graphs that have a large number of edges.
Matrix factorization is a powerful technique that uses a matrices such as an adjacency matrix as input. Common approaches involve using SVD, Laplacian eigenmaps or variants of the two to construct low dimensional representations. Despite reported success, the dependence on matrices like an adjacency matrix creates an issue of scalability as matrices of large networks would take too much memory for a regular computer to operate well. Furthermore, these methods treat all edge types the same, but a possible extension is to incorporate node and edge types as sources of input.
Translational distance models treat edges in a knowledge graph as linear transformations. For example, one such algorithm, TransE [137], treats every node-edge pair as a triplet with head nodes represented as \(\textbf{h}\), edges represented as \(\textbf{r}\), and tail nodes represented as \(\textbf{t}\). These representations are combined into an equation that mimics the iconic word vectors translations (\(\textbf{king} - \textbf{man} + \textbf{woman} \approx \textbf{queen}\)) from the word2vec model [97]. The described equation is shown as follows: \(\textbf{h} + \textbf{r} \approx \textbf{t}\). Starting at the head node (\(\textbf{h}\)), add the edge vector (\(\textbf{r}\)) and the result should be the tail node (\(\textbf{t}\)). TransE optimizes vectors for \(\textbf{h}\), \(\textbf{r}\), \(\textbf{t}\), while guaranteeing the global equation (\(\textbf{h} + \textbf{r} \approx \textbf{t}\)) is satisfied [137]. A caveat to the TransE approach is that it the training steps force relationships to have a one to one mapping, which may not be appropriate for all relationship types.
Wang et al. attempted to resolve the one to one mapping issue by developing the TransH model [138]. TransH treats relations as hyperplanes rather than a regular vector and projects the head (\(\textbf{h}\)) and tail (\(\textbf{t}\)) nodes onto this hyperplane. Following this projection, a distance vector (\(\textbf{d}_{r}\)) is calculated between the projected head and tail nodes. Finally, each vector is optimized while preserving the global equation: \(\textbf{h} + \textbf{d}_{r} \approx \textbf{t}\) [138]. Other effots have built off of the TransE and TransH models [139,140]. In the future, it may be beneficial for these models is to incorporate other types of information such as edge confidence scores, textual information, or edge type information when optimizing these vectors.
Deep learning is a paradigm that uses multiple non-linear transformations to map high dimensional data into a low dimensional space. Many techniques that use deep learning on knowledge graphs are based on word2vec [96,97], a set of approaches that are widely used for natural language processing. The goal of word2vec is to project words onto a low dimensional space that preserves their semantic meaning. Strategies for training word2vec models use one of two neural network architectures: skip-gram and continuous bag of words (CBOW). Both models are feed-forward neural networks, but CBOW models are trained to predict a word given its context while skip-gram models are trained to predict the context given a word [96,97]. Once training has finished, words are now associated with dense vectors that downstream models, such as feed forward networks or recurrent networks, can use for input.
Deepwalk is an early method that represents knowledge graphs in a low dimensional space [141]. The first step of this method is to perform a random walk along a knowledge graph. During the random walk, every generated sequence of nodes is recorded and treated as a sentence in word2vec [96,97]. After every node has been processed, a skip-gram model is trained to predict the context of each node thereby constructing a low dimensional representation of a knowledge graph [141]. A limitation for deepwalk is that the random walk cannot be controlled, so every node has an equal chance to be reached. Grover and Leskovec demonstrated that this limitation can hurt performance when classifying edges between nodes and developed node2vec as a result [142]. Node2vec operates the in the same fashion as deepwalk; however, this algorithm specifies a parameter that lets the random walk be biased when traversing nodes [142]. A caveat to both deepwalk and node2vec is that they ignore information such as edge type and node type. Various approaches have evolved to fix this limitation by incorporating node, edge and even path types when representing knowledge graphs in a low dimensional space [143,144,145,146]. An emerging area of work is to develop approaches that capture both the local and global structure of a graph when constructing this low dimensional space.
Instead of using the word2vec framework, some deep learning approaches use an adjacency matrix as input [96,97]. These approaches use neural networks called autoencoders to generate this low dimensional space [147,148,149]. Autoencoders map input such as an adjacency matrices into a low dimensional space and then determines how to construct this space via reconstructing the same input [150,151]. This generated space represents the nodes and their connectivity structure within a knowledge graph [147,148,149]. Despite the high potential of this approach, this method relies on an adjacency matrix for input which can run into scalability issues as a knowledge graph asymptotically increases in size [152]. Plus, Khosla et al. discovered that approaches akin to node2vec outperformed algorithms using autoencoders when undergoing link prediction and node classification [152]. Overall, the performance of deep learning techniques largely depends upon the structure of nodes and edges within a knowledge graph [152]. Future work should include hybrid models that use both node2vec and autoencoders to construct complementary low dimensional representations of knowledge graphs.
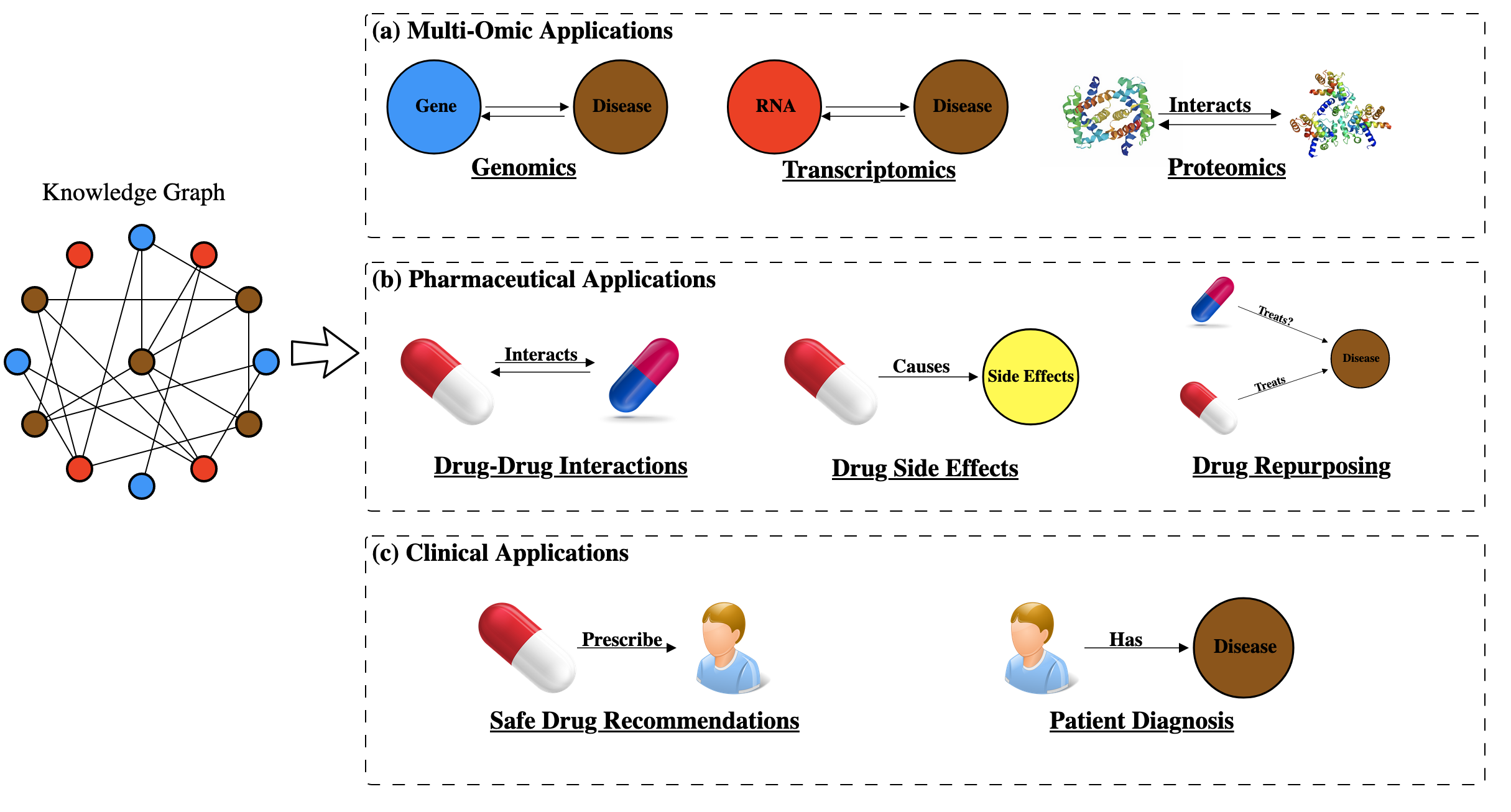
Knowledge graphs have been applied to many biomedical challenges ranging from identifying proteins’ functions [153] to prioritizing cancer genes [154] to recommending safer drugs to patients [155,156] (Figure 5). In this section we review how knowledge graphs are applied in biomedical settings and put particular emphasis on an emerging set of techniques that represent knowledge graphs in a low dimensional space.
Multi-omic applications employ knowledge graphs to study the genome, how genes are expressed in the transcriptome, and how the products of those transcripts interact in the proteome. These graphs are used to establish connections between -omic entities as well as diseases. Tasks in this context include gene-symptom prioritization [157], protein-protein interaction prediction [158,159], and detecting miRNA-disease associations [120]. We focus specifically on multi-omic applications that represent knowledge graphs in a low dimensional space to make connections.
Recommendation systems make use of knowledge graphs to establish links between RNA with disease and proteins with other proteins. Shen et al. used an algorithm called collaborative filtering to establish an association between miRNA and diseases [120]. The authors constructed an miRNA-Disease network using the Human MicroRNA Disease database (HMDD) [160] and generated an adjacency matrix with the rows representing miRNA and the columns representing diseases. This matrix was decomposed into small rectangular matrices using SVD, then these small matrices were used to calculate similarity scores between miRNAs and diseases. High scores implied a high likelihood that a given miRNA had an association with a given disease [120]. Other approaches have built off of Shen et al.’s work by incorporating novel ways to perform matrix factorization [161,162,163] or by integrating machine learning models in conjunction with matrix factorization [164]. These approaches achieved high area under the receiver operating curve (AUROC), but new discoveries have been hard to validate as experiments in this space are costly and time consuming at best [120]. Apart from miRNA, collaborative filtering has been used to predict protein-protein interactions [158,159,165]. Although extensive validation of newly generated candidates may be impractical, it would be helpful to see future efforts in this space include a blinded literature search for prioritized and randomly selected candidates as part of the standard evaluation pipeline.
Knowledge graphs benefited the multi-omics field as a resource for generating novel discoveries. Most approaches to date use matrix factorization and node2vec to project knowledge graph into a low dimensional space, while translational models may be an untapped resource that could aid future efforts. Another area of exploration could be incorporating multiple sources of information such as compounds, anatomic locations or genetic pathways to improve the specificity of findings (i.e., to predict that a protein-protein interaction happens in a specific cell type or tissue).
There are a multitude of examples where knowledge graphs have been applied to identify new properties of drugs. Tasks in this field involve predicting drugs interacting with other drugs [172], identifying molecular targets a drug might interact with [173] and identifying new disease treatments for previously established drugs [174]. In this section we concentrate on applications that apply these graphs to discover new properties of drugs and focus on approaches that use these graphs in a low-dimensional space.
Similar to multi-omic applications recommendation systems have utilized knowledge graphs to infer novel links between drugs and diseases. Dai et al. used collaborative filtering to infer drug-disease associations [173]. The authors constructed a drug-disease network by integrating two bipartite networks: a drug-gene interaction network and a disease-gene interaction network. They integrated both networks under the assumption that drugs associated with a disease interact with the same gene of interest. Following construction, the authors generated an adjacency matrix where rows represent drugs and columns represent diseases. This matrix was decomposed into two small rectangular matrices and these matrices were used to calculate similarity scores between all drugs and all diseases. High values implied a high chance of an association [173]. Related approaches used this technique to infer drug-target interactions [175,176,177] and drug-disease treatments [178,179,180,181,182]. In spite of reported success, these approaches are limited to the drugs and diseases contained in the graph. Combining these approaches with representations of chemical structures might make it possible to one day make predictions about novel compounds.
Applications that discover new properties of drugs have benefited from using knowledge graphs as a resource. Most methods to date use matrix factorization and deep learning techniques to produce a low-dimensional representation. Due to the success of deep learning [189,190] much of the field’s focus has shifted to these techniques; however, a possible extension is to use an ensemble of deep learning techniques and linear methods to improve performance. Plus, another area of exploration is to incorporate information such as pharmaceutical classes for drugs or chemical structure to improve detection power.
Clinical applications that use knowledge graphs are in early stages of development, but the long-term goal is to use analyses of these graphs to aid patient care. Typically, graphs for these applications are constructed from electronic health records (EHR) and nodes represent patients, drugs and diseases while edges represent relationships such as a patient being prescribed a treatment or a patient being diagnosed with a disease [18,191,192,193]. Tasks within this field range from improving patient diagnoses [194,195] to recommending safer drugs for patients [155,195] and we briefly discuss efforts that use knowledge graphs to accomplish such tasks.
In contrast with most applications where node2vec and autoencoder models have become established, deep learning methods in this field have focused on using graph attention models [196]. These models mimic machine translation models [197] and aim to simultaneously represent knowledge graphs in a low dimensional space and perform the task at hand. Choi et al. used a graph attention model to predict patient diagnoses [119]. The authors constructed a directed graph using medical concepts from patient EHR data. This directed graph was fed into a graph attention network and then used to predict a patient’s likelihood of heart failure [119]. Other approaches have used graph attention models to perform clinical tasks such as drug safety recommendations [156] and patient diagnoses [198].
Knowledge graphs have shown promising results when used for clinical applications; however, there is still room for improvement. Most approaches have run into the common problem of missing data within EHR [119,155,156]. Future directions for the field consist of designing algorithms that can fill in this missing data gap or construct models that can take missing data into account.
Knowledge graphs are becoming widely used in biomedicine, and we expect their use to continue to grow. At the moment, most are constructed from databases derived from manual curation or from co-occurrences in text. However, we expect that machine learning approaches will play a key role in quickly bringing new findings into these graphs. Representing these knowledge graphs in a low dimensional space that captures a graph’s local and global structure can enable many downstream machine learning analyses, and methods to capture this structure are an active area of research.
As with any field, rigorous evaluation that can identify key factors that drive success is critical to moving the field forward. In regard to knowledge graphs, evaluation remains hard. Experiments in this context require a significant amount of time and consequently resources [120,157]. Moving from open ended and uncontrolled evaluations that consist of describing findings that are consistent with the literature to blinded evaluations of the literature that corroborate predictions and non-predictions would be a valuable first step. There are also well-documented biases related to node degree and degree distribution that must be considered for accurate evaluation [199]. Furthermore, the diversity of applications hinders the development of a standardized set of expected evaluations.
We anticipate that a fruitful avenue of research will be techniques that can produce low dimensional representations of knowledge graphs that distinguish between multple node and edge types. There are also many different sources of bias that lead to spurious edges or incompleteness, and modeling these may support better representations. It is a promising time for research into the construction and application of knowledge graphs. The peer reviewed literature is growing at an increasing rate and maintaining a complete understanding is becoming increasingly challenging for scientists. One path that scientists can take to maintain complete awareness is to become hyper-focused on specific areas of knowledge graph literature. If advances in how these graphs are constructed, represented, and applied can enable the linking of fields, we may be able to savor the benefits of this detailed knowledge without losing the broader contextual links.
2. Network Embedding Based Recommendation Method in Social Networks
Yufei Wen, Lei Guo, Zhumin Chen, Jun Ma
Companion of the The Web Conference 2018 on The Web Conference 2018 - WWW ’18 (2018) https://doi.org/gf6rtt
DOI: 10.1145/3184558.3186904
3. Open Question Answering with Weakly Supervised Embedding Models
Antoine Bordes, Jason Weston, Nicolas Usunier
arXiv (2014-04-16) https://arxiv.org/abs/1404.4326v1
4. Neural Network-based Question Answering over Knowledge Graphs on Word and Character Level
Denis Lukovnikov, Asja Fischer, Jens Lehmann, Sören Auer
Proceedings of the 26th International Conference on World Wide Web - WWW ’17 (2017) https://doi.org/gfv8hp
DOI: 10.1145/3038912.3052675
6. PhenoGeneRanker: A Tool for Gene Prioritization Using Complete Multiplex Heterogeneous Networks
Cagatay Dursun, Naoki Shimoyama, Mary Shimoyama, Michael Schläppi, Serdar Bozdag
Cold Spring Harbor Laboratory (2019-05-27) https://doi.org/gf6rtr
DOI: 10.1101/651000
7. Biological Random Walks: Integrating heterogeneous data in disease gene prioritization
Michele Gentili, Leonardo Martini, Manuela Petti, Lorenzo Farina, Luca Becchetti
2019 IEEE Conference on Computational Intelligence in Bioinformatics and Computational Biology (CIBCB) (2019-07) https://doi.org/gf6rts
DOI: 10.1109/cibcb.2019.8791472
9. Systematic integration of biomedical knowledge prioritizes drugs for repurposing
Daniel Scott Himmelstein, Antoine Lizee, Christine Hessler, Leo Brueggeman, Sabrina L Chen, Dexter Hadley, Ari Green, Pouya Khankhanian, Sergio E Baranzini
eLife (2017-09-22) https://doi.org/cdfk
DOI: 10.7554/elife.26726 · PMID: 28936969 · PMCID: PMC5640425
11. Towards a definition of knowledge graphs
Lisa Ehrlinger, Wolfram Wöß
SEMANTiCS (2016)
13. DrugBank 5.0: a major update to the DrugBank database for 2018
David S Wishart, Yannick D Feunang, An C Guo, Elvis J Lo, Ana Marcu, Jason R Grant, Tanvir Sajed, Daniel Johnson, Carin Li, Zinat Sayeeda, … Michael Wilson
Nucleic Acids Research (2017-11-08) https://doi.org/gcwtzk
DOI: 10.1093/nar/gkx1037 · PMID: 29126136 · PMCID: PMC5753335
14. A network integration approach for drug-target interaction prediction and computational drug repositioning from heterogeneous information
Yunan Luo, Xinbin Zhao, Jingtian Zhou, Jinglin Yang, Yanqing Zhang, Wenhua Kuang, Jian Peng, Ligong Chen, Jianyang Zeng
Nature Communications (2017-09-18) https://doi.org/gbxwrc
DOI: 10.1038/s41467-017-00680-8 · PMID: 28924171 · PMCID: PMC5603535
17. Bio2RDF: Towards a mashup to build bioinformatics knowledge systems
François Belleau, Marc-Alexandre Nolin, Nicole Tourigny, Philippe Rigault, Jean Morissette
Journal of Biomedical Informatics (2008-10) https://doi.org/frqkq5
DOI: 10.1016/j.jbi.2008.03.004 · PMID: 18472304
19. Constructing biomedical domain-specific knowledge graph with minimum supervision
Jianbo Yuan, Zhiwei Jin, Han Guo, Hongxia Jin, Xianchao Zhang, Tristram Smith, Jiebo Luo
Knowledge and Information Systems (2019-03-23) https://doi.org/gf6v26
DOI: 10.1007/s10115-019-01351-4
20. Feature assisted stacked attentive shortest dependency path based Bi-LSTM model for protein–protein interaction
Shweta Yadav, Asif Ekbal, Sriparna Saha, Ankit Kumar, Pushpak Bhattacharyya
Knowledge-Based Systems (2019-02) https://doi.org/gf4788
DOI: 10.1016/j.knosys.2018.11.020
21. Biological Databases- Integration of Life Science Data
Nishant Toomula, Arun Kumar, Sathish Kumar D, Vijaya Shanti Bheemidi
Journal of Computer Science & Systems Biology (2012) https://doi.org/gf8qcb
DOI: 10.4172/jcsb.1000081
22. COSMIC: somatic cancer genetics at high-resolution
Simon A. Forbes, David Beare, Harry Boutselakis, Sally Bamford, Nidhi Bindal, John Tate, Charlotte G. Cole, Sari Ward, Elisabeth Dawson, Laura Ponting, … Peter J. Campbell
Nucleic Acids Research (2016-11-28) https://doi.org/f9v865
DOI: 10.1093/nar/gkw1121 · PMID: 27899578 · PMCID: PMC5210583
23. COSMIC: the Catalogue Of Somatic Mutations In Cancer
John G Tate, Sally Bamford, Harry C Jubb, Zbyslaw Sondka, David M Beare, Nidhi Bindal, Harry Boutselakis, Charlotte G Cole, Celestino Creatore, Elisabeth Dawson, … Simon A Forbes
Nucleic Acids Research (2018-10-29) https://doi.org/gf9hxg
DOI: 10.1093/nar/gky1015 · PMID: 30371878 · PMCID: PMC6323903
24. Recurated protein interaction datasets
Lukasz Salwinski, Luana Licata, Andrew Winter, David Thorneycroft, Jyoti Khadake, Arnaud Ceol, Andrew Chatr Aryamontri, Rose Oughtred, Michael Livstone, Lorrie Boucher, … Henning Hermjakob
Nature Methods (2009-12) https://doi.org/fgvkmf
DOI: 10.1038/nmeth1209-860 · PMID: 19935838
25. Literature-curated protein interaction datasets
Michael E Cusick, Haiyuan Yu, Alex Smolyar, Kavitha Venkatesan, Anne-Ruxandra Carvunis, Nicolas Simonis, Jean-François Rual, Heather Borick, Pascal Braun, Matija Dreze, … Marc Vidal
Nature Methods (2008-12-30) https://doi.org/d4j62p
DOI: 10.1038/nmeth.1284 · PMID: 19116613 · PMCID: PMC2683745
26. Curation accuracy of model organism databases
I. M. Keseler, M. Skrzypek, D. Weerasinghe, A. Y. Chen, C. Fulcher, G.-W. Li, K. C. Lemmer, K. M. Mladinich, E. D. Chow, G. Sherlock, P. D. Karp
Database (2014-06-12) https://doi.org/gf63jz
DOI: 10.1093/database/bau058 · PMID: 24923819 · PMCID: PMC4207230
27. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders
Joanna S. Amberger, Carol A. Bocchini, François Schiettecatte, Alan F. Scott, Ada Hamosh
Nucleic Acids Research (2014-11-26) https://doi.org/gf8qb6
DOI: 10.1093/nar/gku1205 · PMID: 25428349 · PMCID: PMC4383985
29. Text mining and expert curation to develop a database on psychiatric diseases and their genes
Alba Gutiérrez-Sacristán, Àlex Bravo, Marta Portero-Tresserra, Olga Valverde, Antonio Armario, M. C. Blanco-Gandía, Adriana Farré, Lierni Fernández-Ibarrondo, Francina Fonseca, Jesús Giraldo, … Laura I. Furlong
Database (2017-01-01) https://doi.org/gf8qb5
DOI: 10.1093/database/bax043 · PMID: 29220439 · PMCID: PMC5502359
35. CurEx
Michael Loster, Felix Naumann, Jan Ehmueller, Benjamin Feldmann
Proceedings of the 27th ACM International Conference on Information and Knowledge Management - CIKM ’18 (2018) https://doi.org/gf8qb8
DOI: 10.1145/3269206.3269229
36. Re-curation and rational enrichment of knowledge graphs in Biological Expression Language
Charles Tapley Hoyt, Daniel Domingo-Fernández, Rana Aldisi, Lingling Xu, Kristian Kolpeja, Sandra Spalek, Esther Wollert, John Bachman, Benjamin M Gyori, Patrick Greene, Martin Hofmann-Apitius
Database (2019-01-01) https://doi.org/gf7hm4
DOI: 10.1093/database/baz068 · PMID: 31225582 · PMCID: PMC6587072
37. LocText: relation extraction of protein localizations to assist database curation
Juan Miguel Cejuela, Shrikant Vinchurkar, Tatyana Goldberg, Madhukar Sollepura Prabhu Shankar, Ashish Baghudana, Aleksandar Bojchevski, Carsten Uhlig, André Ofner, Pandu Raharja-Liu, Lars Juhl Jensen, Burkhard Rost
BMC Bioinformatics (2018-01-17) https://doi.org/gf8qb9
DOI: 10.1186/s12859-018-2021-9 · PMID: 29343218 · PMCID: PMC5773052
38. Evaluating the impact of pre-annotation on annotation speed and potential bias: natural language processing gold standard development for clinical named entity recognition in clinical trial announcements
Todd Lingren, Louise Deleger, Katalin Molnar, Haijun Zhai, Jareen Meinzen-Derr, Megan Kaiser, Laura Stoutenborough, Qi Li, Imre Solti
Journal of the American Medical Informatics Association (2014-05) https://doi.org/f5zggh
DOI: 10.1136/amiajnl-2013-001837 · PMID: 24001514 · PMCID: PMC3994857
40. BioSimplify: an open source sentence simplification engine to improve recall in automatic biomedical information extraction.
Siddhartha Jonnalagadda, Graciela Gonzalez
AMIA … Annual Symposium proceedings. AMIA Symposium (2010-11-13) https://www.ncbi.nlm.nih.gov/pubmed/21346999
PMID: 21346999 · PMCID: PMC3041388
41. The EU-ADR corpus: Annotated drugs, diseases, targets, and their relationships
Erik M. van Mulligen, Annie Fourrier-Reglat, David Gurwitz, Mariam Molokhia, Ainhoa Nieto, Gianluca Trifiro, Jan A. Kors, Laura I. Furlong
Journal of Biomedical Informatics (2012-10) https://doi.org/f36vn6
DOI: 10.1016/j.jbi.2012.04.004 · PMID: 22554700
42. Comparative experiments on learning information extractors for proteins and their interactions
Razvan Bunescu, Ruifang Ge, Rohit J. Kate, Edward M. Marcotte, Raymond J. Mooney, Arun K. Ramani, Yuk Wah Wong
Artificial Intelligence in Medicine (2005-02) https://doi.org/dhztpn
DOI: 10.1016/j.artmed.2004.07.016 · PMID: 15811782
47. The BioGRID interaction database: 2013 update
Andrew Chatr-aryamontri, Bobby-Joe Breitkreutz, Sven Heinicke, Lorrie Boucher, Andrew Winter, Chris Stark, Julie Nixon, Lindsay Ramage, Nadine Kolas, Lara O’Donnell, … Mike Tyers
Nucleic Acids Research (2012-11-30) https://doi.org/f4jmz4
DOI: 10.1093/nar/gks1158 · PMID: 23203989 · PMCID: PMC3531226
48. The Comparative Toxicogenomics Database: update 2019
Allan Peter Davis, Cynthia J Grondin, Robin J Johnson, Daniela Sciaky, Roy McMorran, Jolene Wiegers, Thomas C Wiegers, Carolyn J Mattingly
Nucleic Acids Research (2018-09-24) https://doi.org/gf8qb7
DOI: 10.1093/nar/gky868 · PMID: 30247620 · PMCID: PMC6323936
49. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database
Baofeng Jia, Amogelang R. Raphenya, Brian Alcock, Nicholas Waglechner, Peiyao Guo, Kara K. Tsang, Briony A. Lago, Biren M. Dave, Sheldon Pereira, Arjun N. Sharma, … Andrew G. McArthur
Nucleic Acids Research (2016-10-26) https://doi.org/f9wbjs
DOI: 10.1093/nar/gkw1004 · PMID: 27789705 · PMCID: PMC5210516
55. BELMiner: adapting a rule-based relation extraction system to extract biological expression language statements from bio-medical literature evidence sentences
K. E. Ravikumar, Majid Rastegar-Mojarad, Hongfang Liu
Database (2017-01-01) https://doi.org/gf7rbx
DOI: 10.1093/database/baw156 · PMID: 28365720 · PMCID: PMC5467463
57. Construction of phosphorylation interaction networks by text mining of full-length articles using the eFIP system
Catalina O. Tudor, Karen E. Ross, Gang Li, K. Vijay-Shanker, Cathy H. Wu, Cecilia N. Arighi
Database (2015-01-01) https://doi.org/gf8fpt
DOI: 10.1093/database/bav020 · PMID: 25833953 · PMCID: PMC4381107
59. LimTox: a web tool for applied text mining of adverse event and toxicity associations of compounds, drugs and genes
Andres Cañada, Salvador Capella-Gutierrez, Obdulia Rabal, Julen Oyarzabal, Alfonso Valencia, Martin Krallinger
Nucleic Acids Research (2017-05-22) https://doi.org/gf479h
DOI: 10.1093/nar/gkx462 · PMID: 28531339 · PMCID: PMC5570141
63. RLIMS-P 2.0: A Generalizable Rule-Based Information Extraction System for Literature Mining of Protein Phosphorylation Information
Manabu Torii, Cecilia N. Arighi, Gang Li, Qinghua Wang, Cathy H. Wu, K. Vijay-Shanker
IEEE/ACM Transactions on Computational Biology and Bioinformatics (2015-01-01) https://doi.org/gf8fpv
DOI: 10.1109/tcbb.2014.2372765 · PMID: 26357075 · PMCID: PMC4568560
65. PhpSyntaxTree tool
A Eisenbach, M Eisenbach
(2006)
66. Spacy 2: Natural language understanding with bloom embeddings, convolutional neural networks and incremental parsing
Matthew Honnibal, Ines Montani
To appear (2017)
67. STRING v9.1: protein-protein interaction networks, with increased coverage and integration
Andrea Franceschini, Damian Szklarczyk, Sune Frankild, Michael Kuhn, Milan Simonovic, Alexander Roth, Jianyi Lin, Pablo Minguez, Peer Bork, Christian von Mering, Lars J. Jensen
Nucleic Acids Research (2012-11-29) https://doi.org/gf5kcd
DOI: 10.1093/nar/gks1094 · PMID: 23203871 · PMCID: PMC3531103
68. A comprehensive and quantitative comparison of text-mining in 15 million full-text articles versus their corresponding abstracts
David Westergaard, Hans-Henrik Stærfeldt, Christian Tønsberg, Lars Juhl Jensen, Søren Brunak
PLOS Computational Biology (2018-02-15) https://doi.org/gcx747
DOI: 10.1371/journal.pcbi.1005962 · PMID: 29447159 · PMCID: PMC5831415
69. STITCH 4: integration of protein–chemical interactions with user data
Michael Kuhn, Damian Szklarczyk, Sune Pletscher-Frankild, Thomas H. Blicher, Christian von Mering, Lars J. Jensen, Peer Bork
Nucleic Acids Research (2013-11-28) https://doi.org/f5shb4
DOI: 10.1093/nar/gkt1207 · PMID: 24293645 · PMCID: PMC3964996
72. A new method for prioritizing drug repositioning candidates extracted by literature-based discovery
Majid Rastegar-Mojarad, Ravikumar Komandur Elayavilli, Dingcheng Li, Rashmi Prasad, Hongfang Liu
2015 IEEE International Conference on Bioinformatics and Biomedicine (BIBM) (2015-11) https://doi.org/gf479j
DOI: 10.1109/bibm.2015.7359766
74. STRING v10: protein–protein interaction networks, integrated over the tree of life
Damian Szklarczyk, Andrea Franceschini, Stefan Wyder, Kristoffer Forslund, Davide Heller, Jaime Huerta-Cepas, Milan Simonovic, Alexander Roth, Alberto Santos, Kalliopi P. Tsafou, … Christian von Mering
Nucleic Acids Research (2014-10-28) https://doi.org/f64rfn
DOI: 10.1093/nar/gku1003 · PMID: 25352553 · PMCID: PMC4383874
77. BioCreative V CDR task corpus: a resource for chemical disease relation extraction
Jiao Li, Yueping Sun, Robin J. Johnson, Daniela Sciaky, Chih-Hsuan Wei, Robert Leaman, Allan Peter Davis, Carolyn J. Mattingly, Thomas C. Wiegers, Zhiyong Lu
Database (2016) https://doi.org/gf5hfw
DOI: 10.1093/database/baw068 · PMID: 27161011 · PMCID: PMC4860626
82. Extraction of relations between genes and diseases from text and large-scale data analysis: implications for translational research
Àlex Bravo, Janet Piñero, Núria Queralt-Rosinach, Michael Rautschka, Laura I Furlong
BMC Bioinformatics (2015-02-21) https://doi.org/f7kn8s
DOI: 10.1186/s12859-015-0472-9 · PMID: 25886734 · PMCID: PMC4466840
86. Deep learning for pharmacovigilance: recurrent neural network architectures for labeling adverse drug reactions in Twitter posts
Anne Cocos, Alexander G Fiks, Aaron J Masino
Journal of the American Medical Informatics Association (2017-02-22) https://doi.org/gbp9nj
DOI: 10.1093/jamia/ocw180 · PMID: 28339747
87. Semantic Relations in Compound Nouns: Perspectives from Inter-Annotator Agreement
Yadav Prabha, Jezek Elisabetta, Bouillon Pierrette, Callahan Tiffany J., Bada Michael, Hunter Lawrence E., Cohen K. Bretonnel
Studies in Health Technology and Informatics (2017) https://doi.org/ggmk8t
DOI: 10.3233/978-1-61499-830-3-644
88. Cross-Sentence N-ary Relation Extraction with Graph LSTMs
Nanyun Peng, Hoifung Poon, Chris Quirk, Kristina Toutanova, Wen-tau Yih
arXiv (2017-08-12) https://arxiv.org/abs/1708.03743v1
95. Revisiting Unreasonable Effectiveness of Data in Deep Learning Era
Chen Sun, Abhinav Shrivastava, Saurabh Singh, Abhinav Gupta
arXiv (2017-07-10) https://arxiv.org/abs/1707.02968v2
96. Efficient Estimation of Word Representations in Vector Space
Tomas Mikolov, Kai Chen, Greg Corrado, Jeffrey Dean
arXiv (2013-01-16) https://arxiv.org/abs/1301.3781v3
97. Distributed Representations of Words and Phrases and their Compositionality
Tomas Mikolov, Ilya Sutskever, Kai Chen, Greg Corrado, Jeffrey Dean
arXiv (2013-10-16) https://arxiv.org/abs/1310.4546v1
100. Extraction of protein–protein interactions (PPIs) from the literature by deep convolutional neural networks with various feature embeddings
Sung-Pil Choi
Journal of Information Science (2016-11-01) https://doi.org/gcv8bn
DOI: 10.1177/0165551516673485
102. Expanding a Database-derived Biomedical Knowledge Graph via Multi-relation Extraction from Biomedical Abstracts
David N. Nicholson, Daniel S. Himmelstein, Casey S. Greene
Cold Spring Harbor Laboratory (2019-08-08) https://doi.org/gf6qxh
DOI: 10.1101/730085
103. Distant supervision for relation extraction without labeled data
Mike Mintz, Steven Bills, Rion Snow, Dan Jurafsky
Proceedings of the Joint Conference of the 47th Annual Meeting of the ACL and the 4th International Joint Conference on Natural Language Processing of the AFNLP: Volume 2 - ACL-IJCNLP ’09 (2009) https://doi.org/fg9q43
DOI: 10.3115/1690219.1690287
111. Learning protein protein interaction extraction using distant supervision
Philippe Thomas, Illés Solt, Roman Klinger, Ulf Leser
(2011-01)
112. Robust Distant Supervision Relation Extraction via Deep Reinforcement Learning
Pengda Qin, Weiran Xu, William Yang Wang
arXiv (2018-05-24) https://arxiv.org/abs/1805.09927v1
113. DSGAN: Generative Adversarial Training for Distant Supervision Relation Extraction
Pengda Qin, Weiran Xu, William Yang Wang
arXiv (2018-05-24) https://arxiv.org/abs/1805.09929v1
116. Learning language in logic - genic interaction extraction challenge
C. Nédellec
Proceedings of the learning language in logic 2005 workshop at the international conference on machine learning (2005)
118. Concept annotation in the CRAFT corpus
Michael Bada, Miriam Eckert, Donald Evans, Kristin Garcia, Krista Shipley, Dmitry Sitnikov, William A Baumgartner Jr, K Bretonnel Cohen, Karin Verspoor, Judith A Blake, Lawrence E Hunter
BMC Bioinformatics (2012-07-09) https://doi.org/gb8vdr
DOI: 10.1186/1471-2105-13-161 · PMID: 22776079 · PMCID: PMC3476437
119. GRAM: Graph-based Attention Model for Healthcare Representation Learning
Edward Choi, Mohammad Taha Bahadori, Le Song, Walter F. Stewart, Jimeng Sun
arXiv (2016-11-21) https://arxiv.org/abs/1611.07012v3
120. miRNA-Disease Association Prediction with Collaborative Matrix Factorization
Zhen Shen, You-Hua Zhang, Kyungsook Han, Asoke K. Nandi, Barry Honig, De-Shuang Huang
Complexity (2017) https://doi.org/ggmrpm
DOI: 10.1155/2017/2498957
134. Graph embedding on biomedical networks: methods, applications and evaluations
Xiang Yue, Zhen Wang, Jingong Huang, Srinivasan Parthasarathy, Soheil Moosavinasab, Yungui Huang, Simon M Lin, Wen Zhang, Ping Zhang, Huan Sun
Bioinformatics (2019-10-04) https://doi.org/ggmzpf
DOI: 10.1093/bioinformatics/btz718 · PMID: 31584634
137. Translating embeddings for modeling multi-relational data
Antoine Bordes, Nicolas Usunier, Alberto García-Durán, Jason Weston, Oksana Yakhnenko
NIPS (2013)
140. PrTransH: Embedding Probabilistic Medical Knowledge from Real World EMR Data
Linfeng Li, Peng Wang, Yao Wang, Jinpeng Jiang, Buzhou Tang, Jun Yan, Shenghui Wang, Yuting Liu
arXiv (2019-09-02) https://arxiv.org/abs/1909.00672v1
143. struc2vec
Leonardo F. R. Ribeiro, Pedro H. P. Saverese, Daniel R. Figueiredo
Proceedings of the 23rd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining - KDD ’17 (2017) https://doi.org/gd874b
DOI: 10.1145/3097983.3098061
144. metapath2vec
Yuxiao Dong, Nitesh V. Chawla, Ananthram Swami
Proceedings of the 23rd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining - KDD ’17 (2017) https://doi.org/gfsqzn
DOI: 10.1145/3097983.3098036
145. edge2vec: Representation learning using edge semantics for biomedical knowledge discovery
Zheng Gao, Gang Fu, Chunping Ouyang, Satoshi Tsutsui, Xiaozhong Liu, Jeremy Yang, Christopher Gessner, Brian Foote, David Wild, Qi Yu, Ying Ding
arXiv (2018-09-07) https://arxiv.org/abs/1809.02269v3
146. Learning Graph Embeddings from WordNet-based Similarity Measures
Andrey Kutuzov, Mohammad Dorgham, Oleksiy Oliynyk, Chris Biemann, Alexander Panchenko
arXiv (2018-08-16) https://arxiv.org/abs/1808.05611v4
149. Adversarially Regularized Graph Autoencoder for Graph Embedding
Shirui Pan, Ruiqi Hu, Guodong Long, Jing Jiang, Lina Yao, Chengqi Zhang
arXiv (2018-02-13) https://arxiv.org/abs/1802.04407v2
151. Autoencoders, unsupervised learning and deep architectures
Pierre Baldi
Proceedings of the 2011 international conference on unsupervised and transfer learning workshop - volume 27 (2011)
155. Safe Medicine Recommendation via Medical Knowledge Graph Embedding
Meng Wang, Mengyue Liu, Jun Liu, Sen Wang, Guodong Long, Buyue Qian
arXiv (2017-10-16) https://arxiv.org/abs/1710.05980v2
156. GAMENet: Graph Augmented MEmory Networks for Recommending Medication Combination
Junyuan Shang, Cao Xiao, Tengfei Ma, Hongyan Li, Jimeng Sun
Proceedings of the AAAI Conference on Artificial Intelligence (2019-07-17) https://doi.org/ggkm7r
DOI: 10.1609/aaai.v33i01.33011126
157. Heterogeneous network embedding for identifying symptom candidate genes
Kuo Yang, Ning Wang, Guangming Liu, Ruyu Wang, Jian Yu, Runshun Zhang, Jianxin Chen, Xuezhong Zhou
Journal of the American Medical Informatics Association (2018-10-23) https://doi.org/gfg6nr
DOI: 10.1093/jamia/ocy117 · PMID: 30357378
158. Predicting Protein–Protein Interactions from Multimodal Biological Data Sources via Nonnegative Matrix Tri-Factorization
Hua Wang, Heng Huang, Chris Ding, Feiping Nie
Journal of Computational Biology (2013-04) https://doi.org/f4thrx
DOI: 10.1089/cmb.2012.0273 · PMID: 23509857
160. HMDD v3.0: a database for experimentally supported human microRNA–disease associations
Zhou Huang, Jiangcheng Shi, Yuanxu Gao, Chunmei Cui, Shan Zhang, Jianwei Li, Yuan Zhou, Qinghua Cui
Nucleic Acids Research (2018-10-26) https://doi.org/ggmrph
DOI: 10.1093/nar/gky1010 · PMID: 30364956 · PMCID: PMC6323994
164. LWPCMF: Logistic Weighted Profile-based Collaborative Matrix Factorization for Predicting MiRNA-Disease Associations
Meng-Meng Yin, Zhen Cui, Ming-Ming Gao, Jin-Xing Liu, Ying-Lian Gao
IEEE/ACM Transactions on Computational Biology and Bioinformatics (2019) https://doi.org/ggmrpk
DOI: 10.1109/tcbb.2019.2937774 · PMID: 31478868
165. Protein-protein interaction prediction via Collective Matrix Factorization
Qian Xu, Evan Wei Xiang, Qiang Yang
2010 IEEE International Conference on Bioinformatics and Biomedicine (BIBM) (2010-12) https://doi.org/csnv5m
DOI: 10.1109/bibm.2010.5706537
166. A network embedding model for pathogenic genes prediction by multi-path random walking on heterogeneous network
Bo Xu, Yu Liu, Shuo Yu, Lei Wang, Jie Dong, Hongfei Lin, Zhihao Yang, Jian Wang, Feng Xia
BMC Medical Genomics (2019-12) https://doi.org/ggmrpq
DOI: 10.1186/s12920-019-0627-z · PMID: 31865919 · PMCID: PMC6927107
167. Predicting gene-disease associations from the heterogeneous network using graph embedding
Xiaochan Wang, Yuchong Gong, Jing Yi, Wen Zhang
2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM) (2019-11) https://doi.org/ggmrpj
DOI: 10.1109/bibm47256.2019.8983134
171. Detection of protein complexes from multiple protein interaction networks using graph embedding
Xiaoxia Liu, Zhihao Yang, Shengtian Sang, Hongfei Lin, Jian Wang, Bo Xu
Artificial Intelligence in Medicine (2019-05) https://doi.org/ggmrpf
DOI: 10.1016/j.artmed.2019.04.001 · PMID: 31164203
172. Large-scale structural and textual similarity-based mining of knowledge graph to predict drug–drug interactions
Ibrahim Abdelaziz, Achille Fokoue, Oktie Hassanzadeh, Ping Zhang, Mohammad Sadoghi
Journal of Web Semantics (2017-05) https://doi.org/gcrwk3
DOI: 10.1016/j.websem.2017.06.002
173. Matrix Factorization-Based Prediction of Novel Drug Indications by Integrating Genomic Space
Wen Dai, Xi Liu, Yibo Gao, Lin Chen, Jianglong Song, Di Chen, Kuo Gao, Yongshi Jiang, Yiping Yang, Jianxin Chen, Peng Lu
Computational and Mathematical Methods in Medicine (2015) https://doi.org/gb58g8
DOI: 10.1155/2015/275045 · PMID: 26078775 · PMCID: PMC4452507
175. Drug-Target Interaction Prediction with Graph Regularized Matrix Factorization
Ali Ezzat, Peilin Zhao, Min Wu, Xiao-Li Li, Chee-Keong Kwoh
IEEE/ACM Transactions on Computational Biology and Bioinformatics (2017-05-01) https://doi.org/ggmrrp
DOI: 10.1109/tcbb.2016.2530062 · PMID: 26890921
177. Network-based prediction of drug–target interactions using an arbitrary-order proximity embedded deep forest
Xiangxiang Zeng, Siyi Zhu, Yuan Hou, Pengyue Zhang, Lang Li, Jing Li, L Frank Huang, Stephen J Lewis, Ruth Nussinov, Feixiong Cheng
Bioinformatics (2020-01-23) https://doi.org/ggmrrk
DOI: 10.1093/bioinformatics/btaa010 · PMID: 31971579
178. DrPOCS: Drug Repositioning Based on Projection Onto Convex Sets
Yin-Ying Wang, Chunfeng Cui, Liqun Qi, Hong Yan, Xing-Ming Zhao
IEEE/ACM Transactions on Computational Biology and Bioinformatics (2019-01-01) https://doi.org/ggmrrq
DOI: 10.1109/tcbb.2018.2830384 · PMID: 29993698
181. Drug–Disease Association and Drug-Repositioning Predictions in Complex Diseases Using Causal Inference–Probabilistic Matrix Factorization
Jihong Yang, Zheng Li, Xiaohui Fan, Yiyu Cheng
Journal of Chemical Information and Modeling (2014-08-22) https://doi.org/f6hpb4
DOI: 10.1021/ci500340n · PMID: 25116798
184. Scalable and Accurate Drug–target Prediction Based on Heterogeneous Bio-linked Network Mining
Nansu Zong, Rachael Sze Nga Wong, Victoria Ngo, Yue Yu, Ning Li
Cold Spring Harbor Laboratory (2019-02-03) https://doi.org/ggmrrm
DOI: 10.1101/539643
185. Drug Similarity Integration Through Attentive Multi-view Graph Auto-Encoders
Tengfei Ma, Cao Xiao, Jiayu Zhou, Fei Wang
arXiv (2018-04-28) https://arxiv.org/abs/1804.10850v1
187. DrugBank: a knowledgebase for drugs, drug actions and drug targets
David S. Wishart, Craig Knox, An Chi Guo, Dean Cheng, Savita Shrivastava, Dan Tzur, Bijaya Gautam, Murtaza Hassanali
Nucleic Acids Research (2007-11-29) https://doi.org/d3qqpj
DOI: 10.1093/nar/gkm958 · PMID: 18048412 · PMCID: PMC2238889
190. Drug-Drug Interaction Prediction Based on Knowledge Graph Embeddings and Convolutional-LSTM Network
Md. Rezaul Karim, Michael Cochez, Joao Bosco Jares, Mamtaz Uddin, Oya Beyan, Stefan Decker
Proceedings of the 10th ACM International Conference on Bioinformatics, Computational Biology and Health Informatics - BCB ’19 (2019) https://doi.org/ggmrrs
DOI: 10.1145/3307339.3342161
192. Applying linked data principles to represent patient’s electronic health records at Mayo clinic
Jyotishman Pathak, Richard C. Kiefer, Christopher G. Chute
Proceedings of the 2nd ACM SIGHIT symposium on International health informatics - IHI ’12 (2012) https://doi.org/fzm2p7
DOI: 10.1145/2110363.2110415
193. PDD Graph: Bridging Electronic Medical Records and Biomedical Knowledge Graphs via Entity Linking
Meng Wang, Jiaheng Zhang, Jun Liu, Wei Hu, Sen Wang, Xue Li, Wenqiang Liu
arXiv (2017-07-17) https://arxiv.org/abs/1707.05340v2
194. Diagnosis Code Assignment Using Sparsity-Based Disease Correlation Embedding
Sen Wang, Xiaojun Chang, Xue Li, Guodong Long, Lina Yao, Quan Z. Sheng
IEEE Transactions on Knowledge and Data Engineering (2016-12-01) https://doi.org/f9cgtv
DOI: 10.1109/tkde.2016.2605687
195. EMR-based medical knowledge representation and inference via Markov random fields and distributed representation learning
Chao Zhao, Jingchi Jiang, Yi Guan
arXiv (2017-09-20) https://arxiv.org/abs/1709.06908v1
196. Attention Is All You Need
Ashish Vaswani, Noam Shazeer, Niki Parmar, Jakob Uszkoreit, Llion Jones, Aidan N. Gomez, Lukasz Kaiser, Illia Polosukhin
arXiv (2017-06-12) https://arxiv.org/abs/1706.03762v5
197. Neural Machine Translation by Jointly Learning to Align and Translate
Dzmitry Bahdanau, Kyunghyun Cho, Yoshua Bengio
arXiv (2014-09-01) https://arxiv.org/abs/1409.0473v7
198. Learning the Graphical Structure of Electronic Health Records with Graph Convolutional Transformer
Edward Choi, Zhen Xu, Yujia Li, Michael W. Dusenberry, Gerardo Flores, Yuan Xue, Andrew M. Dai
arXiv (2019-06-11) https://arxiv.org/abs/1906.04716v3
199. The probability of edge existence due to node degree: a baseline for network-based predictions
Michael Zietz, Daniel S. Himmelstein, Kyle Kloster, Christopher Williams, Michael W. Nagle, Blair D. Sullivan, Casey S. Greene
Manubot (2020-03-05) https://greenelab.github.io/xswap-manuscript/
0000-0003-0002-5761 ·
danich1
0000-0001-8713-9213 ·
cgreene ·
greenescientist
![Figure 1: A metagraph (schema) of the heterogeneous network used in the Rephetio project [9]. This undirected network depicts pharmacological and biomedical information. The nodes (circles) represent entities and edges (lines) depict relational information between two entities.](https://raw.githubusercontent.com/dhimmel/rephetio/f02d44fde7eeef0ffdca0800e0b43c48d800c86d/figure/metagraph.png)
![Figure 2: A visualization of a constituency parse tree using the following sentence: “BRCA1 is associated with breast cancer” [65]. This type of tree has the root start at the beginning of the sentence. Each word is grouped into subphrases depending its correlating part of speech tag. For example, the word “associated” is a past participle verb (VBN) that belongs to the verb phrase (VP) subgroup.](images/figures/constituency_parse_tree_example.png)
![Figure 3: A visualization of a dependency parse tree using the following sentence: “BRCA1 is associated with breast cancer” [66]. For these type of trees the root begins with the main verb of the sentence. Each arrows represents the dependency shared between two words. For example, the dependency between BRCA1 and associated is nsubjpass, which stands for passive nominal subject. This means that “BRCA1” is the subject of the sentence and it is being referred to by the word “associated”.](images/figures/dependency_parse_example.png)